How To Count Sigma And Pi Bonds
How To Count Sigma And Pi Bonds. In acetylene, the ##”c≡c”## triple bond consists of a sigma bond and two pi bonds. A double or triple bond consists of both sigma and pi bonds.
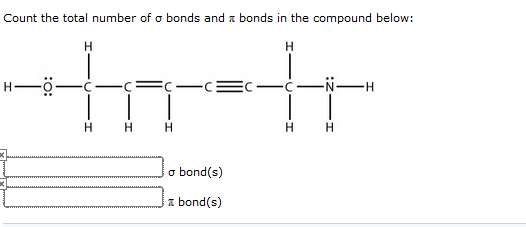
We say you will this kind of what are sigma and pi bonds graphic could possibly be the most trending subject considering we share it in google pro or facebook. There are as many sigma bonds as bonds (of any kind, simple,double or triple). It explains how to calculate the number of sigma and pi bonds in a mole.
A Triple Bond Consists Of Two Pi Bonds And One Sigma Bond.
Trick to find number of sigma and pi bonds. Can a pi bond hold 4 electrons? Y = number of hydrogen atoms and p = number of π bonds/double bonds.
Hence The Given Compound Contains \[10\] Sigma Bonds And \[3\] Pi Bonds.
A π bond is the same strength as a σ bond. When we come to no. It explains how to calculate the number of sigma and pi bonds in a mole.
Triple Bond = 1 Sigma Bond + 2 Pi Bonds.
Of pi bonds in each carbon in benzene, one hybridised orbital will overlap with other carbon axially to form pi bonds. Is c2h2 a triple bond? There is an easy way to count sigma and pi bonds.
Postby Irenegi2I » Mon Nov 30, 2020 7:36 Am.
2 number of electrons in the antibonding molecular orbital: Triple bonds are comprised of one sigma bond and two pi bonds. Hence all single bonds are sigma bonds.
In General, Single Bonds Between Atoms Are Always Sigma Bonds.
Single bond = 1 sigma bond. Double bonds have one each, and triple bonds have one sigma bond and two pi bonds. I calculated how many single/double/triple bonds are there.
Post a Comment for "How To Count Sigma And Pi Bonds"